- Product Details
Keywords
- Valacyclovir hydrochloride supplier
- Valacyclovir hydrochloride China
- Valacyclovir hydrochloride 98%
Quick Details
- ProName: Valacyclovir hydrochloride 98% supplie...
- CasNo: 124832-27-5
- Molecular Formula: C13H21ClN6O4
- Appearance: powder
- Application: API
- PackAge: 7.5KG
- Port: shanghai
- ProductionCapacity: Metric Ton/Day
- Purity: 98%
- Storage: Room temperature
- Transportation: by air or by sea
- LimitNum: 0 Metric Ton
Superiority
High quality product, competitive price and best service.
Details
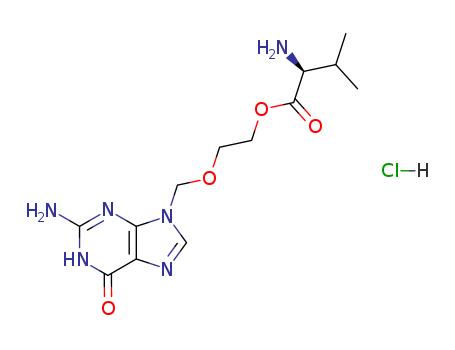
1.Introduction of Valacyclovir hydrochloride
The Valacyclovir hydrochloride, with the CAS registry number 124832-27-5, is also known as 9-((2-Hydroxy-ethoxy)methyl)guanine L-valine ester hydrochloride. It belongs to the product categories of Active Pharmaceutical Ingredients; Nucleotides and Nucleosides; Bases & Related Reagents; Intermediates & Fine Chemicals; Nucleotides; Pharmaceuticals; API's; Valacyclovir. This chemical's molecular formula is C13H21ClN6O4 and molecular weight is 360.80. What's more, its systematic name is 2-[(2-Amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate hydrochloride (1:1). Its classification codes are: (1)Anti-Infective Agents; (2)Antiviral; (3)Antiviral Agents. This chemical is used in the management of HSV and VZV infections and the prophylaxis of HCMV infections.
2.Physical properties of Valacyclovir hydrochloride
(1)ACD/LogP: -0.883; (2)# of Rule of 5 Violations: 2; (3)ACD/LogD (pH 5.5): -3.08 ; (4)ACD/LogD (pH 7.4): -1.39; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 2.43; (9)#H bond acceptors: 10; (10)#H bond donors: 5; (11)#Freely Rotating Bonds: 9; (12)Polar Surface Area: 92.5 ?2; (13)Flash Point: 309.7 °C; (14)Enthalpy of Vaporization: 87.84 kJ/mol; (15)Boiling Point: 588.4 °C at 760 mmHg; (16)Vapour Pressure: 7.95E-14 mmHg at 25°C.
3.structure of Valacyclovir hydrochloride
(1)SMILES: Cl.O=C(OCCOCn1c2N\C(=N/C(=O)c2nc1)N)[C@@H](N)C(C)C
(2)Std. InChI: InChI=1S/C13H20N6O4.ClH/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10
(19)17-13(15)18-11(9)20;/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20);1H/t8-;/m0./s1
(3)Std. InChIKey: ZCDDBUOENGJMLV-QRPNPIFTSA-N
4.Pharmacodynamics of Valacyclovir hydrochloride
Valacyclovir hydrochloride is a prodrug and synthetic purine nucleoside analogue with inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV). Valaciclovir is almost completely converted to acyclovir and L-valine. The inhibitory activity of valaciclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, which is then converted into acyclovir diphosphate and triphosphate by cellular enzymes. Acyclovir is selectively converted to the active triphosphate form by cells infected with herpes viruses.
5.Mechanism of action of Valacyclovir hydrochloride
Valaciclovir is phosphorylated by viral thymidine kinase to acyclovir triphosphate (the active metabolite) which then inhibits herpes viral DNA replication by competitive inhibition of viral DNA polymerase, and by incorporation into and termination of the growing viral DNA chain. When used as a substrate for viral DNA polymerase, acyclovir triphosphate competitively inhibits dATP leading to the formation of 'faulty' DNA. This is where acyclovir triphosphate is incorporated into the DNA strand replacing many of the adenosine bases. This results in the prevention of DNA synthesis, as phosphodiester bridges can longer to be built, destabilizing the strand.
6.Safty information of Valacyclovir hydrochloride
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing.