- Product Details
Keywords
- Pyrene supplier
- Pyrene China
- Pyrene 98%
Quick Details
- ProName: Pyrene 98% supplier in China
- CasNo: 129-00-0
- Molecular Formula: C16H10
- Appearance: powder
- Application: fine chemical, Organic synthesis, pest...
- PackAge: 25kg
- Port: shanghai
- ProductionCapacity: Metric Ton/Day
- Purity: 98%
- Storage: Sealed storage
- Transportation: by air or by sea
- LimitNum: 1 Kilogram
Superiority
we have 3 OEM factories, 2 R&D labs. Aily is striving to improve reseach and development, meet social and customers needs with a unifying strategic idea.
Details
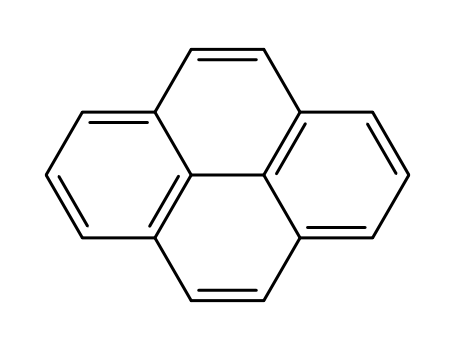
1.Introduction of Pyrene
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This colourless solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds.
2.Properties of Pyrene
(1)ACD/LogP: 5.004 (2)# of Rule of 5 Violations: 1
(3)ACD/LogD (pH 5.5): 5.00 (4)ACD/LogD (pH 7.4): 5.00
(5)ACD/BCF (pH 5.5): 3739.36 (6)ACD/BCF (pH 7.4): 3739.36
(7)ACD/KOC (pH 5.5): 12560.35 (8)ACD/KOC (pH 7.4): 12560.35
(9)Index of Refraction: 1.852 (10)Molar Refractivity: 72.464 cm3
(11)Molar Volume: 161.978 cm3 (12)Polarizability: 28.727 10-24cm3
(13)Surface Tension: 59.4039993286133 dyne/cm (14)Density: 1.249 g/cm3
(15)Flash Point: 168.777 °C (16)Enthalpy of Vaporization: 62.986 kJ/mol
(17)Boiling Point: 403.998 °C at 760 mmHg
3.Structure descriptors of Pyrene
SMILES:
c1cc2ccc3cccc4c3c2c(c1)cc4
Std. InChI:
InChI=1S/C16H10/c1-3-11-7-9-13-5-2-6-14-10-8-12(4-1)15(11)16(13)14/h1-10H
Std. InChIKey:
BBEAQIROQSPTKN-UHFFFAOYSA-N
4.Applications of Pyrene
Pyrene and its derivatives are used commercially to make dyes and dye precursors, for example pyranine and naphthalene-1,4,5,8-tetracarboxylic acid. Its derivatives are also valuable molecular probes via fluorescence spectroscopy, having a high quantum yield and lifetime (0.65 and 410 nanoseconds, respectively, in ethanol at 293 K). Its fluorescence emission spectrum is very sensitive to solvent polarity, so pyrene has been used as a probe to determine solvent environments. This is due to its excited state having a different, non-planar structure than the ground state. Certain emission bands are unaffected, but others vary in intensity due to the strength of interaction with a solvent.