- Product Details
Keywords
- 21245-02-3 price
Quick Details
- ProName: 2-Ethylhexyl 4-dimethylaminobenzoate
- CasNo: 21245-02-3
- Molecular Formula: C17H27NO2
- Appearance: colorless to yellowish liquid
- Application: ultraviolet absorber in cosmetics
- PackAge: 180kg/drum
- Port: shanghai
- ProductionCapacity: /Day
- Purity: 99%
- Storage: storage in shady and cool warehouse
- Transportation: as the regulation of chemicals
- LimitNum: 0 Metric Ton
Superiority
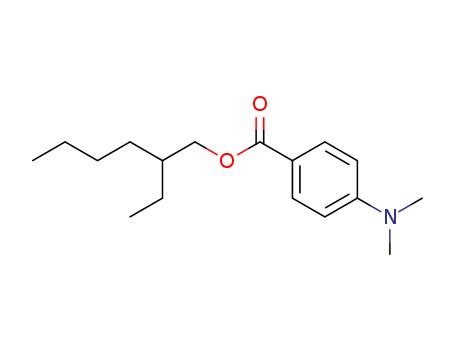
1. Introduction of2-Ethylhexyl 4-dimethylaminobenzoate
The 2-Ethylhexyl 4-dimethylaminobenzoate, with its CAS NO21245-02-3, is a kind ofcol
Details
1. Introduction of 2-Ethylhexyl 4-dimethylaminobenzoate
The 2-Ethylhexyl 4-dimethylaminobenzoate, with its CAS NO 21245-02-3, is a kind of colorless to yellowish liquid. It has synonyms of Benzoicacid, p-(dimethylamino)-, 2-ethylhexyl ester (8CI);2-Ethylhexyl4-(N,N-dimethylamino)benzoate;2-Ethylhexyl N,N-dimethyl-p-aminobenzoate;2-Ethylhexyl p-(dimethylamino)benzoate;4-(Dimethylamino)benzoic acid 2-ethylhexyl ester;Arlatone UVB;EHDAB;EsacureEHA;Escalol 507;Eusolex 6007;Octyl dimethyl PABA;QuantacureEHA and 2-Ethylhexyl 4-dimethylaminobenzoate.
2. Properties of 2-Ethylhexyl 4-dimethylaminobenzoate
ACD/LogP: 5.412 # of Rule of 5 Violations: 1
ACD/LogD (pH 5.5): 5.41 ACD/LogD (pH 7.4): 5.41
ACD/BCF (pH 5.5): 7636.25 ACD/BCF (pH 7.4): 7642.04
ACD/KOC (pH 5.5): 20934.46 ACD/KOC (pH 7.4): 20950.33
#H bond acceptors: 3 #Freely Rotating Bonds: 9 Polar Surface Area: 29.54 2
Index of Refraction: 1.514 Molar Refractivity: 84.728 cm3
Molar Volume: 281.241 cm3 Polarizability: 33.589 10-24cm3
Surface Tension: 36.2360000610352 dyne/cm Density: 0.986 g/cm3
Flash Point: 121.713 °C Enthalpy of Vaporization: 63.138 kJ/mol
Boiling Point: 382.909 °C at 760 mmHg
3. Structure descriptors of 2-Ethylhexyl 4-dimethylaminobenzoate
IUPAC Name: 2-ethylhexyl 4-(dimethylamino)benzoate
InChI: InChI=1S/C17H27NO2/c1-5-7-8-14(6-2)13-20-17(19)15-9-11-16(12-10-15)18(3)
4/h9-12,14H,5-8,13H2,1-4H3InChIKey: WYWZRNAHINYAEF-UHFFFAOYSA-N
Canonical SMILES : CCCCC(CC)COC(=O)C1=CC=C(C=C1)N(C)C