- Product Details
Keywords
- 24424-99-5 supplier
- Di-tert-butyl dicarbonate China
- Di-tert-butyl dicarbonate 99%min
Quick Details
- ProName: Di-tert-butyl dicarbonate 99%min suppl...
- CasNo: 24424-99-5
- Molecular Formula: C10H18O5
- Appearance: Colourless liquid and white crystal
- Application: widely used in biochemical, cosmetic, ...
- Port: shanghai
- ProductionCapacity: Metric Ton/Day
- Purity: 99%min
- Storage: sealed storage in shady and cool wareh...
- Transportation: by air or by sea
- LimitNum: 1 Kilogram
Superiority
we have 3 OEM factories, 2 R&D labs. Aily is striving to improve reseach and development, meet social and customers needs with a unifying strategic idea.
Details
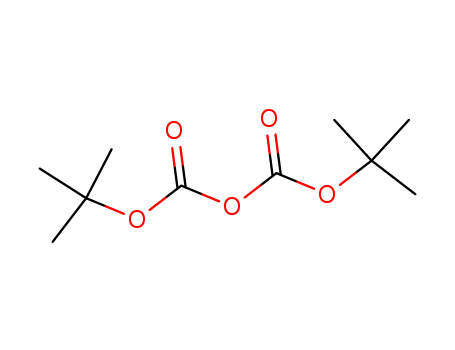
1. Introduction of Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have otherwise affected the amine functional group. The t-BOC can later be removed from the amine using acids. Thus, t-BOC serves as a protective group, for instance in solid phase peptide synthesis. It is unreactive to most bases and nucleophiles, allowing for an orthogonal Fmoc protection.
2. Properties of Di-tert-butyl dicarbonate
ACD/LogP: 2.631 ACD/LogD (pH 5.5): 2.63 ACD/LogD (pH 7.4): 2.63
ACD/BCF (pH 5.5): 58.78 ACD/BCF (pH 7.4): 58.78
ACD/KOC (pH 5.5): 642.73 ACD/KOC (pH 7.4): 642.73
#H bond acceptors: 5 #Freely Rotating Bonds: 6 Polar Surface Area: 61.83 2
Index of Refraction: 1.431 Molar Refractivity: 53.604 cm3
Molar Volume: 207.026 cm3 Polarizability: 21.25 10-24cm3
Surface Tension: 31.2080001831055 dyne/cm Density: 1.054 g/cm3
Flash Point: 103.65 °C Enthalpy of Vaporization: 47.261 kJ/mol
Boiling Point: 235.829 °C at 760 mmHg Vapour Pressure: 0.0489999987185001 mmHg at 25°C
3. Structure descriptors of Di-tert-butyl dicarbonate
IUPAC Name: tert-butyl (2-methylpropan-2-yl)oxycarbonyl carbonate
InChI: InChI=1S/C10H18O5/c1-9(2,3)14-7(11)13-8(12)15-10(4,5)6/h1-6H3
InChIKey: DYHSDKLCOJIUFX-UHFFFAOYSA-N
Canonical SMILES : CC(C)(C)OC(=O)OC(=O)OC(C)(C)C
4. Preparation of Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is inexpensive, so it is usually purchased. Classically, this compound is prepared from tert-butanol, carbon dioxide, phosgene, using DABCO as a base:

This route is currently employed commercially by manufacturers in China and India. European and Japanese companies use the reaction of sodium tert-butylate with carbon dioxide, catalysed by p-toluenesulfonic acid or methanesulfonic acid. This process involves a distillation of the crude material yielding a very pure grade.
Boc anhydride is also available as a 70% solution in toluene or THF. Since boc anhydride is a low-melting solid, having the reagent in a solution simplifies storage and handling.
5. Other uses
The synthesis of 6-acetyl-1,2,3,4-tetrahydropyridine, an important bread aroma compound from 2-piperidone was accomplished using t-boc anhydride. (See Maillard reaction). The first step in this reaction sequence is the formation of the carbamate from the reaction of the secondary amine with boc anhydride in acetonitrile with DMAP as a catalyst.