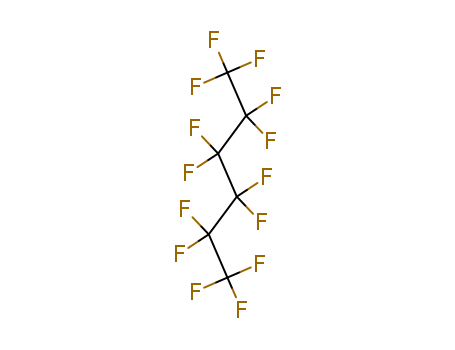- Product Details
Keywords
- Perfluorohexane supplier
- Perfluorohexane China
- Perfluorohexane 98%
Quick Details
- ProName: Perfluorohexane 98% supplier in China
- CasNo: 355-42-0
- Molecular Formula: C6F14
- Appearance: colourless liquid
- Port: shanghai
- ProductionCapacity: Metric Ton/Day
- Purity: 98%
- Storage: storage in shady and cool warehouse
- Transportation: by air or by sea
- LimitNum: 1 Kilogram
Superiority
we have 3 OEM factories, 2 R&D labs. Aily is striving to improve reseach and development, meet social and customers needs with a unifying strategic idea.
Details
1. Introduction of Perfluorohexane
Perfluorohexane (C6F14) or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low temperature applications due to its low boiling point of 56°C and freezing point of -90 °C. It is odorless and colorless.
It is one of the Fluorinert brand solvents/coolants. It is used as a solvent and coolant. In medical imaging it is used as a contrast agent.
Perfluorohexane is considered biologically inert and chemically stable. Like other fluorocarbon based liquids, perfluorohexane dissolves gases including oxygen from the air to a higher concentration than is found in other liquids. This effect is due to the nature of the fluorine atom causing low intermolecular forces between perfluorohexane molecules, which allows "space" for gas molecules to partition into the liquid. Animals can be submerged in a bath of perfluorohexane without drowning, as there is sufficient oxygen available in the solvent to allow respiration to continue. This has led to the experimental use of perfluorohexane in treating burn victims, as their lungs can be filled with either perfluorohexane vapor or in extreme cases liquid perfluorohexane, allowing breathing to continue without the problems normally seen with pulmonary edema that sometimes occur when the inside of the lungs have been burnt e.g. by inhalation of hot smoke.
2. Properties of Perfluorohexane
ACD/LogP: 5.092 # of Rule of 5 Violations: 1
ACD/LogD (pH 5.5): 5.09 ACD/LogD (pH 7.4): 5.09
ACD/BCF (pH 5.5): 4360.81 ACD/BCF (pH 7.4): 4360.81
ACD/KOC (pH 5.5): 14021.53 ACD/KOC (pH 7.4): 14021.53
#Freely Rotating Bonds: 3 Polar Surface Area: 0 2
Index of Refraction: 1.248 Molar Refractivity: 31.933 cm3
Molar Volume: 204.133 cm3 Polarizability: 12.659 10-24cm3
Surface Tension: 11.3319997787476 dyne/cm Density: 1.656 g/cm3
Flash Point: 1.223 °C Enthalpy of Vaporization: 28.803 kJ/mol
Boiling Point: 57.781 °C at 760 mmHg Vapour Pressure: 227.876007080078 mmHg at 25°C
3. Structure descriptors of Perfluorohexane
SMILES:FC(F)(C(F)(F)C(F)(F)F)C(F)(F)C(F)(F)C(F)(F)F
Std. InChI:InChI=1S/C6F14/c7-1(8,3(11,12)5(15,16)17)2(9,10)4(13,14)6(18,19)20
Std. InChIKey:ZJIJAJXFLBMLCK-UHFFFAOYSA-N