- Product Details
Keywords
- Ceftizoxime sodium supplier
- Ceftizoxime sodium China
- Ceftizoxime sodium injection
Quick Details
- ProName: Ceftizoxime sodium injection supplier ...
- CasNo: 68401-82-1
- Molecular Formula: C13H12N5NaO5S2
- Appearance: powder
- Application: API
- PackAge: 7.5KG
- Port: shanghai
- ProductionCapacity: Metric Ton/Day
- Purity: 98%
- Storage: Room temperature
- Transportation: ordinary goods
- LimitNum: 0 Metric Ton
Superiority
High quality product, competitive price and best service.
Details
1. Introduction of Diethyl fluoromalonate
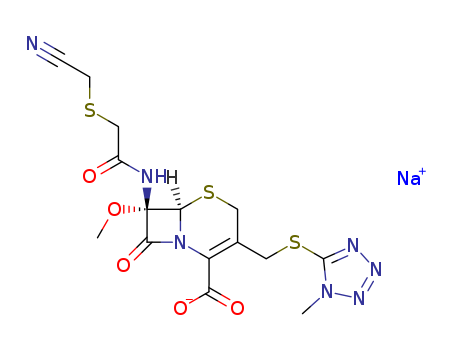
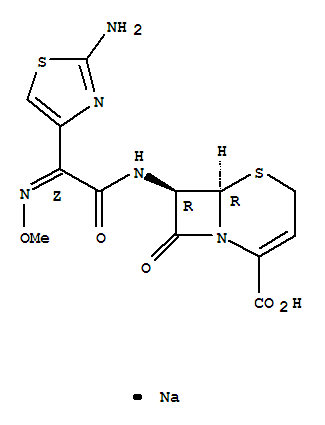
Diethyl fluoromalonate with the CAS registry number 68401-82-1, is also known as 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-, monosodiumsalt, (6R,7R)-. Its systematic name is sodium (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. Its classification codes are: (1)Antibacterial; (2)Antibiotics; (3)Drug / Therapeutic Agent; (4)Reproductive Effect.
2. Basic Information of Diethyl fluoromalonate
It is a semisynthetic cephalosporin antibiotic which can be administered intravenously or by suppository. The drug is highly resistant to a broad spectrum of beta-lactamases and is active against a wide range of both aerobic and anaerobic gram-positive and gram-negative organisms.
1) Properties
Physical properties of Diethyl fluoromalonate is: (1)ACD/LogP: 0.59; (2)# of Rule of 5 Violations: 1; (3)#H bond acceptors: 10; (4)#H bond donors: 4; (5)#Freely Rotating Bonds: 5; (6)Polar Surface Area: 169.18 ?2.
2) Descriptors of Structure
You can still convert the following datas into molecular structure:
(1)SMILES: [Na+].O=C2N1/C(=C\CS[C@@H]1[C@@H]2NC(=O)C(=N\OC)/c3nc(sc3)N)C([O-])=O
(2)Std. InChI: InChI=1S/C13H13N5O5S2.Na/c1-23-17-7(5-4-25-13(14)15-5)9(19)16-8-10(20)18-6(12(21)22)2-3-24-11(8)18;/h2,4,8,11H,3H2,1H3,(H2,14,15)(H,16,19)(H,21,22);/q;+1/p-1/b17-7-;/t8-,11-;/m1./s1
(3)Std. InChIKey: ADLFUPFRVXCDMO-LIGXYSTNSA-M
3) Toxicity
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | > 3200mg/kg (3200mg/kg) |
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) CARDIAC: CARDIOMYOPATHY INCLUDING INFARCTION |
Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
| mouse | LD50 | intraperitoneal | 8930mg/kg (8930mg/kg) |
PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES |
Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
| mouse | LD50 | intravenous | 5150mg/kg (5150mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 24, Pg. 905, 1982. | |
| mouse | LD50 | oral | > 10gm/kg (10000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. | |
| mouse | LD50 | subcutaneous | > 10gm/kg (10000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. | |
| rabbit | LDLo | intravenous | > 3200mg/kg (3200mg/kg) |
BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" |
Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
| rabbit | LDLo | oral | > 3200mg/kg (3200mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. | |
| rat | LD50 | intraperitoneal | 8130mg/kg (8130mg/kg) |
PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES |
Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
| rat | LD50 | intravenous | 5570mg/kg (5570mg/kg) |
PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES |
Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
| rat | LD50 | oral | > 10gm/kg (10000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. | |
| rat | LD50 | subcutaneous | > 10gm/kg (10000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 1669, 1980. |
4) Side effects
Ceftizoxime may cause side effects. If you are administering ceftizoxime into a muscle, it may be mixed with lidocaine (Xylocaine) to reduce pain at the injection site. Tell your health care provider if any of these symptoms are severe or do not go away: stomach pain; upset stomach; vomiting.